|
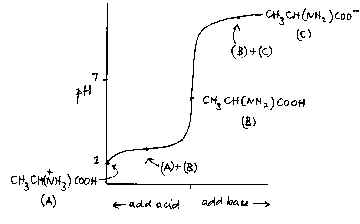
The buffering action of amino acids such as alanine, CH3CH(NH2)COOH, is sometimes referred to in A level syllabuses. The nature of this action is not usually made clear, however, and is often confused with the fact that these compounds are amphiprotic, that is can accept or donate protons. An aqueous solution of alanine has a pH not far off neutrality. It is certainly true that it can accept protons:
+ and it can donate them: CH3CH(NH2)COOH + H2O —> CH3CH(NH2)COO - + H3O+ In both cases, though, the pH changes quite considerably; it is only when enough acid
has been added to give a mixture of CH3CH(NH2)COOH and A similar argument can be made for the addition of base; in this case the buffer mixture is CH3CH(NH2)COOH and CH3CH(NH2)COO - . As with all buffer mixtures, there must be an acid and its conjugate base present for the buffering action to happen. |