
|
The following extract is taken from Kekule’s original paper in which he proposed the ring structure of benzene: Annalen der Chemie, 137: 129 – 196 (1865). Comments are referred to by superscripts and are collected at the end.
STUDIES ON AROMATIC COMPOUNDS
The theory of the atomicity1 of the elements, and especially the knowledge of carbon as a tetratomic2 element, has made possible in recent years in a very satisfactory way the explanation of the atomistic3 constitution of a great many carbon compounds, particularly those which I have called fatty bodies.4 Until now; so far as I know, no one has attempted to apply these views to the aromatic compounds. When I developed my views on the tetratomic nature of carbon seven years ago, I indicated in a note that I had already formed an opinion on this subject, but I had not considered it suitable to develop the idea further. Most chemists who have since written on theoretical questions have left this subject untouched; some stated directly that the composition of aromatic compounds could not be explained by the theory of atomicity; others assumed the existence of a hexatomic group formed by six carbon atoms, but they did not try to find the method of combination of these carbon atoms, nor to give an account of the conditions under which this group could bind six monatomic atoms.
In order to give an account of the atomistic constitution of aromatic compounds, it is necessary to take into consideration the following facts:
1. All aromatic compounds, even the simplest, are proportionally richer in carbon than the analogous compounds in the class of the fatty bodies.
2. Among the aromatic compounds, just as in the fatty bodies, there are numerous homologous substances, i.e., those whose differences of composition can be expressed by n CH2.
3. The simplest aromatic compound contains at least six atoms of carbon.
4. All alteration products of aromatic substances show a certain family similarity, they belong collectively to the group of "aromatic compounds." In more deeply acting reactions, it is true, one part of carbon is often eliminated, but the chief product contains at least six atoms of carbon (benzene, quinone, chloranil, carbolic acid, hydroxyphenic acid, picric acid, etc.). The decomposition stops with the formation of these products if complete destruction of the organic group does not occur.
These facts obviously lead to the conclusion that in all aromatic substances there is contained one and the same atom group, or, if you wish, a common nucleus which consists of six carbon atoms. Within this nucleus the carbon atoms are certainly in close combination or in more compact arrangement. To this nucleus, then, more carbon atoms can add and, indeed, in the same way and according to the same laws as in the case of the fatty bodies.
It is next necessary to give an account of the atomic constitution of this nucleus. Now this can be done very easily by the following hypothesis, which, on the now generally accepted view that carbon is tetratomic, explains in such a simple manner that further development is scarcely necessary
If many carbon atoms can unite with one another, then it can also happen that one affinity unit of one atom can bind one affinity unit of the neighbouring atom. As I have shown earlier, this explains homology and in general the constitution of the fatty bodies.
It can now be further assumed that many carbon atoms are thus linked together, that they are always bound through two affinity units; it can also be assumed that the union occurs alternately through first one and then two affinity units. The first and the last of these views could be expressed by somewhat the following periods:
1/1, 1/1, 1/1, 1/1 etc.
1/1, 2/2,1/1, 2/2 etc.The first law of symmetry of union of the carbon atoms explains the constitution of the fatty bodies, as already mentioned; the second leads to an explanation of the constitution of aromatic substances, or at least of the nucleus which is common to all these substances.
If it is accepted that six carbon atoms are linked together according to this law of symmetry, a group is obtained which, if it is considered as an open chain, still contains eight nonsaturated affinity units. If another assumption is made, that the two carbon atoms which end the chain are linked together by one affinity unit, then there is obtained a closed chain (a symmetrical ring) which still contains six free affinity units5.
From this closed chain now follow all the substances which are usually called aromatic compounds. The open chain occurs in quinone, in chloranil, and in the few substances which stand in close relation to both. I leave these bodies here without further consideration; they are proportionately easy to explain. It can be seen that they stand in close relation with the aromatic substances, but they still cannot truly be counted with the group of aromatic substances.
In all aromatic substances there can be assumed to be a common nucleus; it is the closed chain C6A6 (where A means an unsaturated affinity or affinity unit).
The six affinity units of this nucleus can be saturated by six monatomic elements. They can also all, or at least in part, be saturated by an affinity of a polyatomic element, but this latter must then be joined to other atoms, and so one or more side chains are produced, which can be further lengthened by linking themselves with other elements.
A saturation of two affinity units of the nucleus by an atom of a di-atomic element or a saturation of three affinity units by an atom of a triatomic element is not possible in theory. Compounds of the molecular formula C6H4O, C6H4S, C6H3N are thus unthinkable; if bodies of these compositions exist, and if the theory is correct, the formulas of the first two must be doubled, that of the third tripled.
Comments:
1 atomicity: what we would now call the valency.
2 tetratomic: that is four-valent.
3 atomistic: the idea that substances are made up from atoms joined together; what we would call a molecule.
4 fatty bodies: the alkanes, alkenes, etc – what we would now call aliphatic compounds.
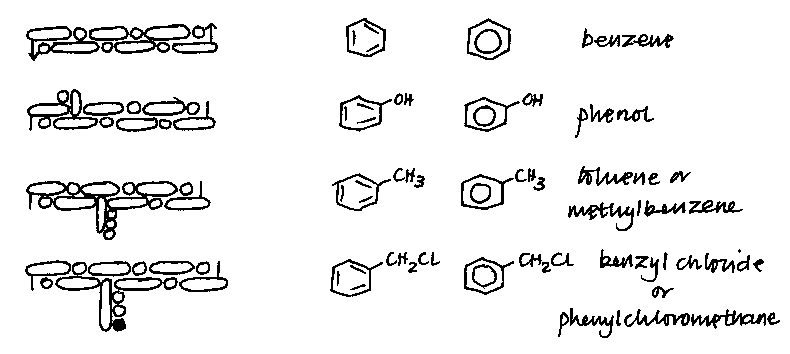
5 At this point Kekule refers to a table of his graphic representations of the molecules. The original does not reproduce well; four of them, together with their modern equivalents, are given below. Kekule’s original footnote is:
In order to make the views developed here more understandable than can be done by words alone, I have gathered "graphic formulas " for many of the substances mentioned here (in Table II). The ideas which can be expressed by these formulas are now so widely known that I need not discuss them again in detail. I have used the same type of graphic formulas that I used in 1859 when for the first time I developed further my views on the atomistic constitution of molecules. This form has been accepted with alterations scarcely worth noting by Wurtz (Lecons de philosophie chimique); it seems to me it offers certain advantages over the recent modifications of Loschmidt and of Crum-Brown. For an understanding of the table, I need only mention that I have represented the closed chain C6A6 as a straight line, thus open, with the dashes denoting the terminal affinities which are to be understood as affinity units linking the opposite ends.
 |