

| Chirality: A chiral molecule is one that is not superimposable on its mirror image; it has the property of rotating the plane of polarisation of plane-polarised monochromatic light that is passed through it. This phenomenon is called optical activity. Many A level syllabuses deal with only one origin of chirality, that is a single carbon atom bearing four different substituent groups. The impression given is that this arrangement is the only condition for chirality, which is not true.
The necessary and sufficient condition… A chiral molecule is one that is not superimposable on its mirror image. Not only is this the best definition – it is short and to the point – but it is the definition. All the other stuff about asymmetric carbon atoms (whatever that means) or four different groups, applies only with certain constraints. Non-superimposability on the mirror image is a necessary and sufficient condition for chirality; no exception has ever been found. The non-superimposability can come about in a number of ways, and need not involve a chiral centre, or even organic molecules at all. Some examples are amusing; since amusement is by far the best reason to do any Chemistry, here are some examples, after a brief excursion into the topic of optical activity. The examples are from March (1).
Optical activity: The degree of rotation of the plane of polarisation of plane-polarised monochromatic light by a chiral compound depends on the path length the light traverses, the concentration of the compound (if it is in solution), the compound itself – and the wavelength of the light. It is not widely promulgated that the amount of rotation a particular sample gives is wavelength dependent, a phenomenon called optical rotatory dispersion. The light usually used for the determination of optical activity is sodium light; at other wavelengths, the rotation will be different, may be zero, and may even reverse in direction compared with the rotation given with sodium light.
|
|
| Tertiary
amines: Some tertiary amines might be thouht to be chiral; the structure is pyramidal and if all three groups about the nitrogen are different then the mirror images appear to be non-superimposable (right). However, if the X, Y, Z groups are independent, then no chirality is shown. This is because the molecules flip inside out very rapidly, in the case of ammonia at a frequency of 2 x 1011 Hz. Amines are slower, but still do not permit resolution into two enantiomers. In order to prevent inversion the nitrogen atom has to be part of a three membered ring and also be connected to an atom that has at least one unshared pair of electrons. The compound 1-chloro-2,2-dimethylaziridine has these properties and has been resolved into its enantiomers. |
A tertiary amine and its apparently non-superimposable mirror image
1-chloro-2,2-dimethylaziridine |
| Molecules
with restricted rotation. Restricted rotation about a single double bond is
well-known as a potential source of geometric isomerism, though it isn't the only one.
Restricted rotation can also give rise to chirality. |
|
| Biphenyls consist of two benzene rings joined by a single bond. If each ring has large substituents on either side of this bond (the 2,6- and 2'-6'- positions) then steric hindrance will prevent rotation. If the substituent groups are different, then the molecule will be chiral. Such enantiomers are called atropisomers, and an example is shown at right. | 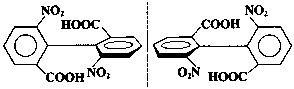 Atropisomers |
Restricted rotation is also shown by allenes, compounds with two double bonds side-by-side. Such bonds are called cumulated, as distinct from alternate double-single-double or conjugated bonds. Allenes are not planar, the groups being in two perpendicular planex; they are chiral only if both sides are unsymmetrically substituted. Allenes with odd numbers of cumulated bonds are not chiral; those with even numbers are. |
|
Restricted rotation can also be found in spiranes, compounds having two rings with one carbon atom in common. This makes the rings perpendicular, and suitable substitution gives rise to chirality. So too can an exocyclic double bond. The compounds at right display these features and are chiral. |
A spirane (top), and an exocylic double bond leading to chirality |
| Chirality
due to a helical shape. Molecules that are helices can be right- or left-handed
and are therefore non-superimposable on their mirror image. The molecule does not have to
have a complete turn of the helix to be chiral. |
hexahelicene - a spiral molecule |
| In trans-cyclooctene the carbon chain
must lie above the double bond on one side and below it on the other, this leading to
chirality.
|
trans-cyclooctene |
| Heptalene has two fused 7-membered rings and is not flat. Its twisted structure makes it chiral, but heptalene itself cannot be resolved because the two forms rapidly interconvert. Bulky substituents slow this process, however, and in the case of the molecule shown the two enantiomers have been isolated. |
A resolvable heptalene derivative |
| Restricted
rotation of other types: There is a wide variety of compounds that show restricted rotation which in consequence are chiral. There are some interesting structures here. |
|
| Paracyclophanes are compounds having a benzene ring within a larger ring of methylene groups. If substituted on the benzene ring, the molecule can be chiral, as is the example shown with ten methylene groups in the large ring. The layered cyclophane to its right is also chiral. |
|
| Metallocenes are compounds that have a metal atom
sandwiched between two rings, usually cyclopentadiene or benzene. If the rings are
suitably substitututed, then the molecule is chiral. Another example shown is the p-bonded complex between fumaric acid and iron tetracarbonyl. |
|
| Cyclooctatetraene is a tub-shaped molecule; its 1,2,3,4-tetramethyl derivative is chiral. So, from rather subtle causes, is 2,5-dideuteriobarrelene. |
|
| Perchlorotriphenylamine (the 'perchloro' bit derives from the replacement of all of the hydrogen atoms with chlorine) is heavily sterically hindered and the molecule is in the shape of a propellor. |
perchlorotriphenylamine |
| Perhaps you have at some time cut a strip of paper, glued one end, and then joined the two ends with a half-twist: the Mobius Strip. You can then convince yourself using a pencil that this strip has only one surface. I think it's highly amusing that chemists have done the same thing with a molecule (2). Note that the bonds are not O-O bonds, but each has a -CH2CH2- group between them. These are omitted for clarity. |
|
Bibliography.
1 March, J., Advanced Organic Chemistry: Wiley, 4th ed. 1992.
2 Walber, Richards & Haltiwangler, J. Am.Chem. Soc. 104, 3219, 1982.