|
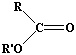
Esters are compunds formed from the reaction between alcohols and acids. The word 'ester' alone now signifies by common usage that the acid is an organic acid, but inorganic acids can also form esters - ATP is an example well-known to biology students, being a phosphate ester. Even halogenoalkanes can be regarded as inorganic esters, of alcohols and hydrochloric acid. This section is, however, confined to esters of organic acids RCOOR' where R can be hydrogen or an organic group:
Esters in the food industry
Esters are widely used for flavourings; many are 'nature-identical', that is synthetic versions of the esters present in the fruit. Fruit flavours are very complex, though, often arising from many different compounds, some of which are present in small quantities.
|
|
Ethyl methanoate (ethyl formate): rum flavouring |
|
|
Propyl pentanoate (n-propyl n-valerate): pineapple flavouring |
|
|
Ethyl butanoate (ethyl butyrate): apple odour |
|
|
Octyl ethanoate (n-octyl acetate): orange odour |
Fats and oils
Fats and oils are tri-esters of glycerol,
propan-1,2,3-triol, with carboxylic acids. These are sometimes called fatty
acids owing to their presence in fats. The difference between oils and fats lies
in their melting temperatures rather than in any fundamental structural
difference. The constituent compounds include: tetradecanoic acid CH3(CH2)12COOH;
hexadecanoic acid, CH3(CH2)14COOH;
octadeca-9,12-dienoic acid (linoleic acid),
CH3(CH2)4(CH=CHCH2)2(CH2)6COOH;
octadeca-9-enoic acid (oleic acid), CH3(CH2)7CH=CH(CH2)7COOH;
propan-1,2,3-triol (glycerol), HOCH2CH(OH)CH2OH.
Margarine:
Margarine contains:
|
|
|
Soft margarines contain a higher proportion of oils and therefore a higher proportion of unsaturated fatty acids than hard margarines. Spreads contain less oil and fat and more water. The composition of a margarine will depend on the climate where it is to be sold.
Margarine manufacture:
|
|
|
Further reading:
The Essential Chemical Industry: Chemical Industry Information Centre, 4th ed., 1999.
Chemistry contents Applied Organic Chemistry Home Page