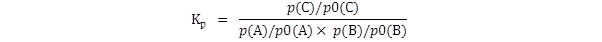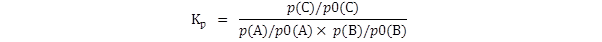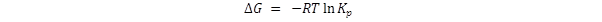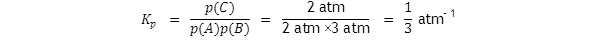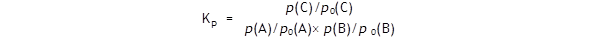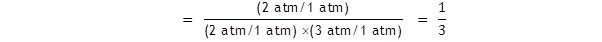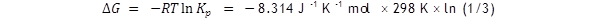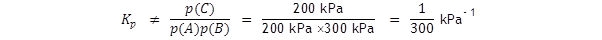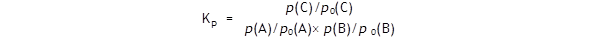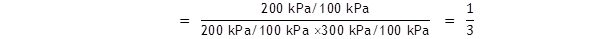|
Before you read the following, here is a caution. Exam Boards continue to ask on
occasions what the units of an equilibrium constant are. You should
continue to answer such questions as you have been taught to do, even
though, as this article shows, they do not have units. The fault lies in the way that the equilibrium constant is defined at A level. This article also shows how calculations must be performed if the standard pressure used is 100 kPa rather than 1 atm.
Standard pressures and the effect on calculations of
Kp.
The new A level specifications for 2015 (first assessment 2017) uniformly
use a standard pressure of 100 kN m – 2
or 100 kPa in their definition of
standard states. This replaces the value of 1 atm which has been used for a
very long time. This decision is not without a knock-on effect in
thermodynamic calculations, especially on how equilibrium constants should
be expressed. Edexcel, OCR, Edexcel and Pre-U specifications all include
Kp, the equilibrium
constant in terms of partial pressure.
The definition of the equilibrium constant for homogeneous gas-phase
reactions has been misrepresented in A level for years. It is time that this
is recognised and corrected with calculations of the Gibbs function ΔG from equilibrium constants now appearing in A level work. If exam
boards are intent on making the standard pressure 100 kN m – 2
or 100 kPa, as has been recommended
by IUPAC to replace the previous value of
Kp
is used for gas mixtures. Equilibrium constants in terms of concentration,
Kc , are not affected in quite the same way
because the standard state for a solution has not been changed from 1 mol dm
– 3. There is a point to be made later about units, however.
Consider the gaseous equilibrium
The equilibrium constant Kp
is generally defined thus:
where p(A),
p(B) and
p(C) are the
equilibrium partial pressures of
the three gases.
If this is the expression which is to be used,
then the partial pressures must be
in atmospheres. If they are not then the numerical value of
Kp
will be wrong.
The definition of
Kp comes from
thermodynamic arguments which it is not appropriate to give here. However
the values
p(A),
p(B) and
p(C) are not quite what they
seem.
In the words of a definitive text, Denbigh (1971 - full reference below):
It will be immediately clear that if all the
p0(X)
are 1 atm then there is no numerical
effect on the value
Since each term
p(X)/p0(X)
is a ratio of two pressures, it is a number.
This means that
Kp
has no units,
since all the terms on the right are numbers - which will surprise many of
you who have been asked to calculate
Kp’s units in examination questions.
Kp
is a number.
The use of 1 atm as the standard pressure simplifies the expression for
Kp but has
unfortunately led to the belief that it has (or can have) units; initially
the 1 atm was omitted but understood, and now is often forgotten.
One of the uses of Kp
is to calculate the Gibbs free energy change through the expression
The logarithm function operates on numbers only; it does not operate on
physical quantities. Therefore
Kp
must be a number, not some value with units such as atm – 1 . To
make Kp a number
requires that the standard pressure be included in the
Kp expression.
Examples using different values for the standard pressure.
Consider the homogeneous gaseous equilibrium already mentioned:
1
With a standard pressure of 1 atm.
Let us assume that the equilibrium partial pressures are
p(A)
= 2
atm,
p(B) = 3
atm
and p(C) = 2
atm.
The standard A level text will have it that
The value of ln (1/3) is – 1.1, so ΔG
= + 2.72 kJ mol –
1
.
2
With a standard pressure of 100 kN m – 2 or 100 kPa.
The equilibrium partial pressures are now, for the same system as above,
p(A)
= 200 kPa,
p(B)
= 300 kPa
and p(C) = 200 kPa.
If you write an expression for
Kp
without including the standard pressure you will not get the true value
; note the ‘not equal to’ symbol!
This would obviously not give the same value for ΔG
as in the first example above.
We must include the standard
state pressure to get the true equilibrium constant:
This gives the same answers for
Kp
and ΔG
as in example 1.
If the standard pressure is to be taken as 100 kPa, as it is in all AS level
specifications where the standard state is being discussed, then any
calculation of Kp at
A2 must involve it if the correct result is to be achieved - unless for this
purpose only a standard pressure of 1 atm is adopted.
The equilibrium constant in terms of concentration,
Kc
The situation is the same here in that the concentrations used in the
expression for Kc
should all be divided by the standard state concentration, which is 1 mol dm
-3. This does not change the numerical value but again it gives a number, as
it must do if the logarithm function is to be applied to it.
Reference Denbigh,
KG, ‘The Principles of Chemical Equilibrium’. Cambridge University Press, 3rd
ed. 1971. |